The imminent launch of drug serialization is generating much interest and anticipation in the healthcare sector. This measure aims to strengthen patient safety by preventing the circulation of counterfeit or poor-quality medicines on the market.
Pharmaceutical laboratories are adapting to this new regulation and implementing serialization systems in their factories. They will also need to provide the National Agency for Medicines and Health Products Safety (ANSM) with information on serialized products to guarantee their traceability.
Drug Serialization
The challenge of drug serialization is to guarantee the authenticity and traceability of pharmaceutical products to prevent counterfeiting and improve patient safety. This will also help fight illegal medicine trade and improve supply chain management.
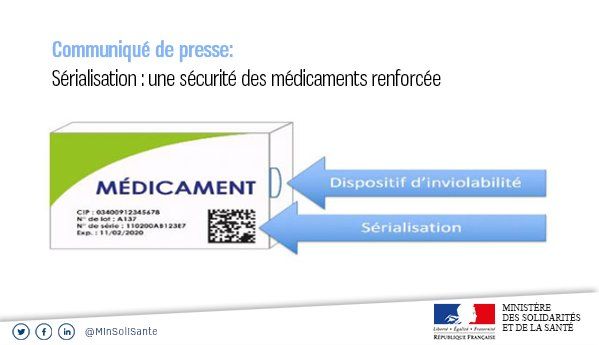
Drug serialization consists of assigning a unique number to each medicine box. This number will be printed on the packaging composed of a barcode called Datamatrix code, which includes information about the product and its manufacturer.
This code will be scanned at each stage of the supply chain, from production to dispensing in hospital pharmacies (PUI) or community pharmacies, thus allowing tracking of each medicine box.
Mandatory information on a medicine box for serialization are:
- CIP;
- Batch number;
- Expiration date;
- Unique serial number;
- Anti-tampering device.
This measure was adopted in response to numerous cases of medicine counterfeiting that have emerged in recent years, endangering patient health. According to the World Health Organization, nearly 10% of medicines worldwide would be counterfeits, causing more than 100,000 deaths each year.
Implementation Date
Drug serialization is a subject of great interest for healthcare professionals and patients. The implementation date of this new regulation has been set for February 9, 2019. This date will mark a turning point for the pharmaceutical industry in France with the entry into force of drug serialization. This measure will strengthen patient safety by guaranteeing the authenticity and integrity of dispensed products.
Directive 2011/62/EU
Directive 2011/62/EU, also known as the Falsified Medicines Directive, aims to strengthen supply chain security by preventing the circulation of falsified medicines on the European market. It establishes requirements for medicine identification and traceability, as well as rules for distribution and online sale of pharmaceutical products.
Drug serialization was validated in consultation at the European level through the adoption of Directive 2011/62/EU on serialization and identification of prescription medicines. This directive was adopted by European Union member states after thorough consultation with stakeholders, including pharmaceutical companies and regulatory authorities.
Read also: “PharmaliZr, First Drug Serialization Software”
The PharmaliZr Solution
PharmaliZr is the ideal solution for drug serialization. The software allows controlling the origin and quality of pharmaceutical products you receive and dispense to your patients. With PharmaliZr, you save time and secure your pharmacy.
To discover our simple and efficient solution, simply contact us. Do not hesitate to request more information, a personalized quote or a free demonstration.
Consult our pricing directly on our website.
