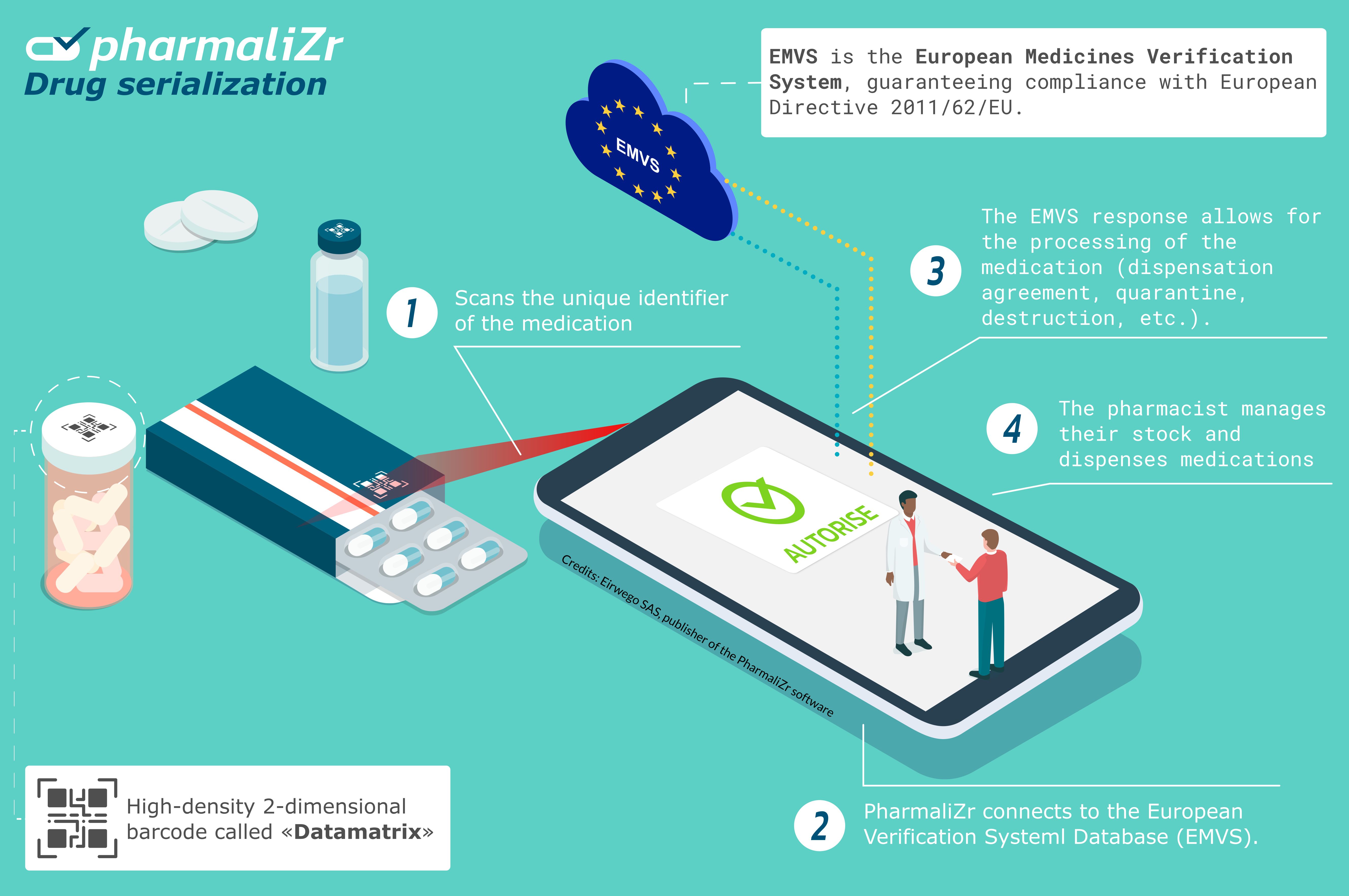
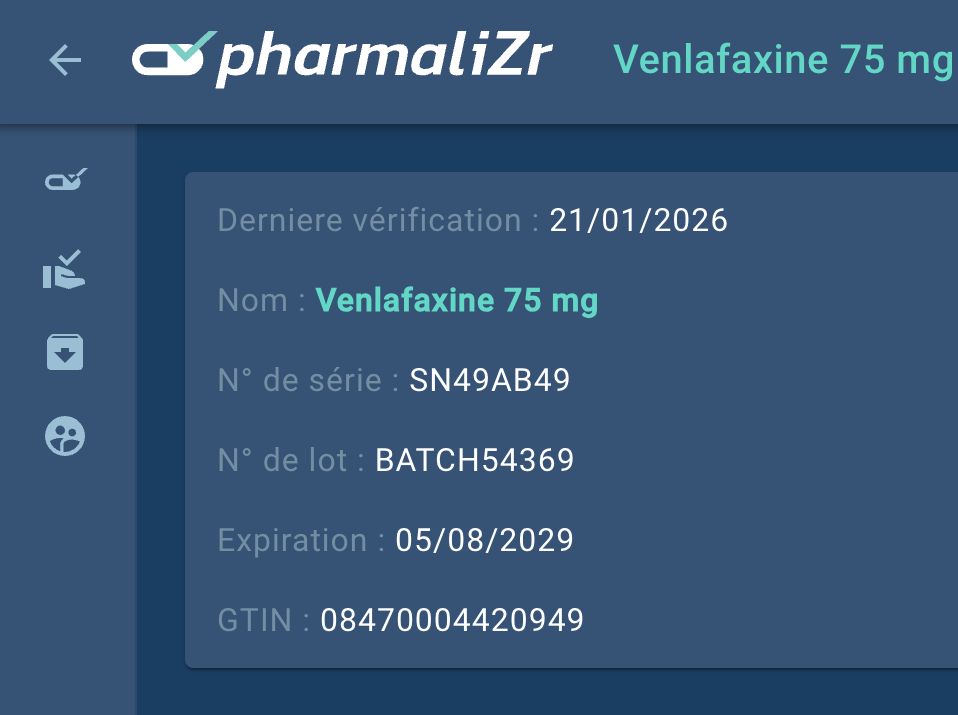
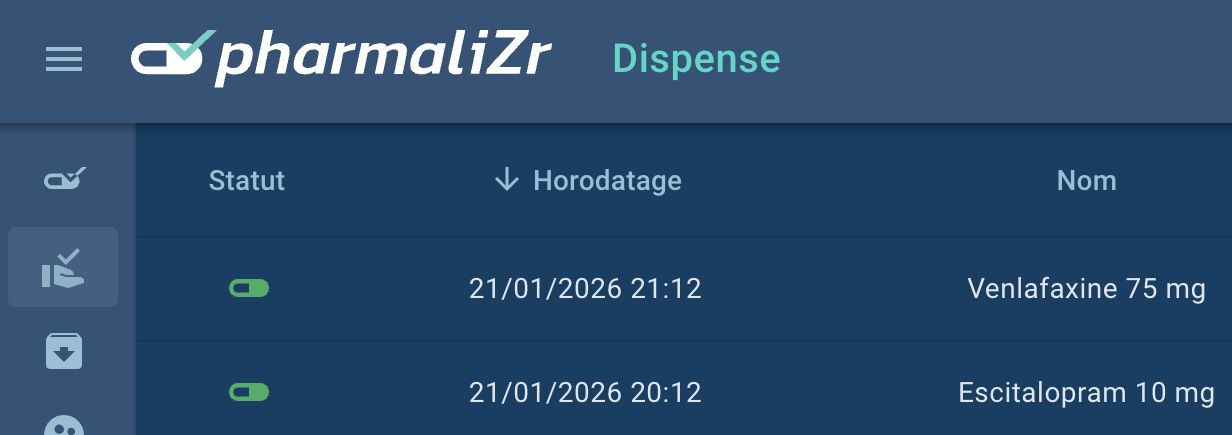
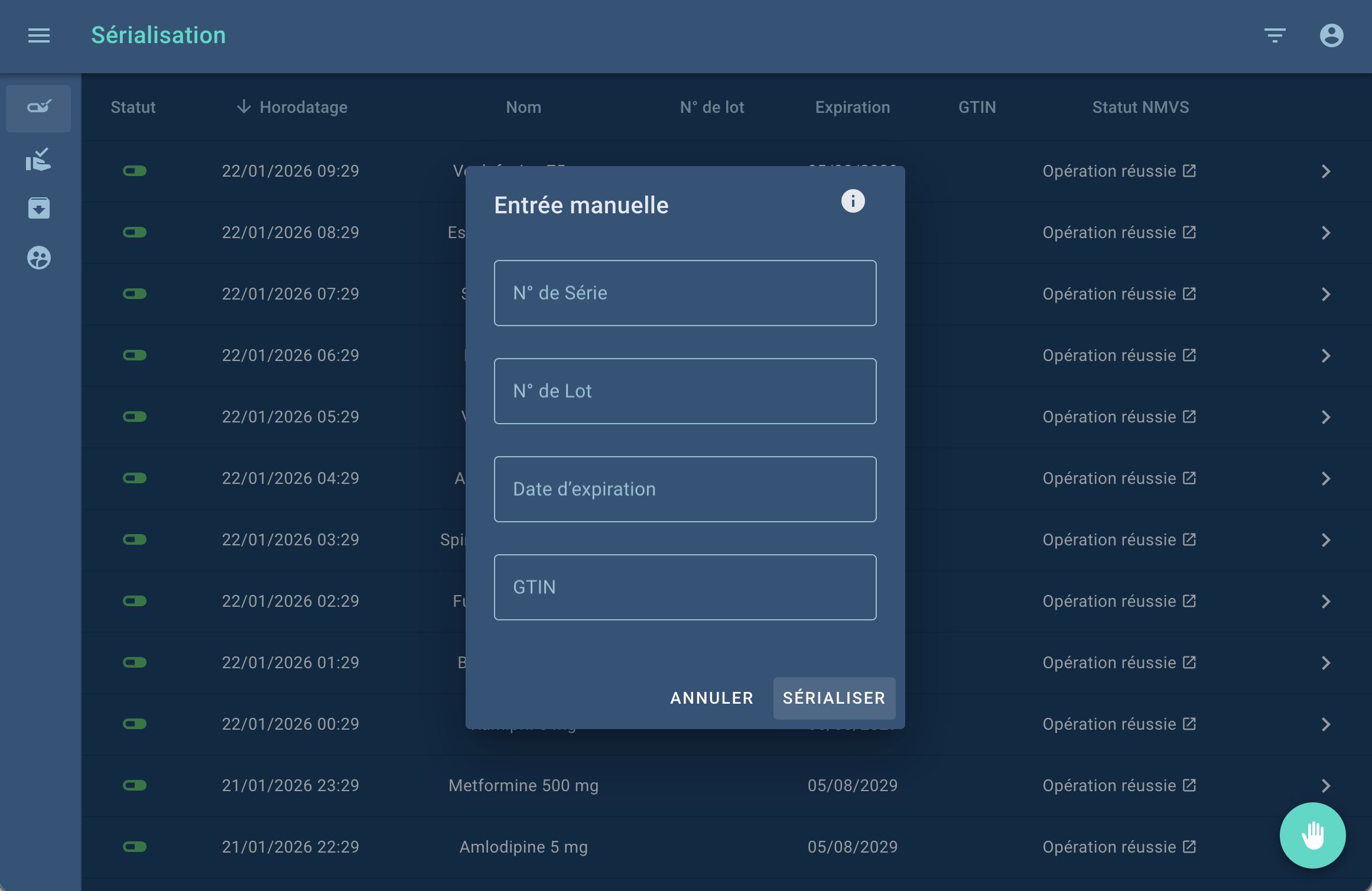
PharmaliZr Integrates the UGAP Market
PharmaliZr has joined the UGAP market, the French public purchasing center that guarantees advantageous prices and simplified payment conditions. By subscribing to the UGAP market, you benefit from the PharmaliZr solution without going through a call for tenders.
PharmaliZr
Eirwego Team